Law of action of mass
1) When a chemical reaction is complete:
When practically all the reagent is scomparso in order to give place to the product of the same reaction.
2) That relationship is between the free energy DG and the equilibrium of one chemical reaction:
Independently from the situation it begins them the system always spontaneously stretches to carry itself to the same composition of equilibrium, which the minimal value of the free energy corresponds.
3) How much is worth DG for one reaction in equilibrium:
It is worth 0 like for all the processes in equilibrium.
4) Enounce the law of action of mass:
When a reaction to constant temperature catches up the equilibrium, it comes defined one constant thermodynamics of equilibrium that in practical represents the yield of the reaction, that is the relationship between products and reagents once that the reaction has been arrested.
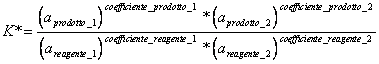
where to it is the activity of the substance correspondent to the ideal concentration.
* for ideal gases to = pressure
* for not ideal gases to = f * p where f it is the activity coefficient
* for solid and pure liquids the activity is unitary.
* for one ideal solution to = concentration.
* for a not ideal solution to = f * where f it is the activity coefficient.
5) Enounce the constant of equilibrium based on the concentration:

it comes used in the reactions that have place in gaseous phase and for the reactions in solution.
6) In which occasion K * and Kc they coincide:
In all those cases in which the activity it coincides with the concentration, like as an example for the gases that follow the ideal behavior (to low pressure) and for the diluted solutions a lot. It is from noticing that only K* is rigorously constant to every insensitive temperature and to pressure variations goddesses reagents and products.
7) Which constant of equilibrium it comes used in the reactions that interest aeriform:
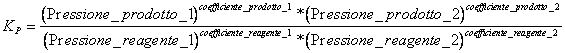
Naturally the substances must be taken in consideration only that in the reaction are introduced to the gaseous state.
8) Which relation alloy Kc and Kp :
Kp = Kc (RT)Dn where Dn is the difference between n° of wharves of the products and the n° of wharves of reagents
One obtains observing that c = massa/volume = n/V and for a gas = partialP / RT, replacing in the Kc obtains the esito.
In this R case size * K is worth 0,0821 (l * Atm/)
9) Enounce the constant of equilibrium in terms of fractions molars:
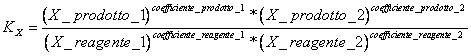
it comes used in the reactions that have place in gaseous phase and for the reactions in solution.
10) Which relation alloy KX and KP :
KP = KX * (PTOTAL )Dn where Dn is the difference between n° of wharves of the products and the n° of wharves of reagents
11) In that occasion KP, KC, KX coincides:
When the reaction happens without change of the n° of wharves of the gaseous substances.
12) That relation is between the constants of equilibrium and the yield of one reaction:
a) if k > > 1 then the reaction has the equilibrium moved to right that is towards the products.
b) if k < < 1 then the reaction has the equilibrium moved on the left that is towards reagents.
13) How much is worth the constant of equilibrium for the inverse reaction to that one considered:
It is the mutual one of the calculate constant of equiibrio for the direct reaction.
14) How much is worth the equilibrium constant if the reaction for n is multiplied:
The constant new of equilibrium is equal to the old constant elevated to the power n-esima.
15) How much is worth the constant of equilibrium for the reaction sum of 2 reactions:
It is the product of the constants of the 2 added reactions.
16) Enounce the constant of equilibrium in function della variation of free energy by means of the relation isoterma of Van' t Hoff:
DG° = - R T ln K
where K can be one whichever of the constants of equilibrium in how much the relation is only worth to pressure and constant temperature. R turns out equal to 1,98 cal/(K*mole) and DG° will turn out expressed in cal/size therefore if the interested wharves are more of one will be necessary to multiply DG° for their number only in such a way will be able to know the variation of determined free energy from the reaction.
17) How much is worth DG° for the reaction: 2 NOCl " 2 NOT Cl2 :
DG° = 2G°f () G°f (Cl2) - 2G°f (NOCl) and not being G°f (element in its state standard) = 0 then
DG° = 2G°f (NO) - 2G°f (NOCl)
18) Describe the relation isocora of Van' t Hoff in shape differentiates them that alloy the constant of equilibrium to the temperature:
![]()
is observed:
a) if DH0 < 0 the reaction is exothermic and the equilibrium constant decreases to growing of the temperature.
b) if DH0 = 0 the reaction is termoneutrale and the equilibrium constant is independent from the temperature.
c) if DH0 > 0 the reaction is endothermic and the equilibrium constant grows to growing of the temperature.
19) Describe the relation isocora of Van' t Hoff in integral shape that alloy the constant of equilibrium to the temperature:
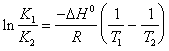
In it R turns out equal to 1,98 cal/(K*mole) and DH° will turn out expressed in cal/size.
To notice that this expression is valid single considering small intervals of temperature, in the which case DH0 it can be considered constant
20) As the isocora is transformed integral of Van' t Hoff for equilibriums between is made of a system to a single member:
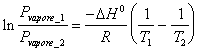
This why in the systems to a single K memberp the activity is equal to the single vapor tension in how much for the liquid is unitary. Analogous for Kc .
21) How much is worth DH° for the reaction: 2 NOCl " 2 NOT Cl2 :
DH° = " 2H°f () H°f (Cl2) - 2H°f (NOCl) and not being H°f (element in its state standard) = 0 then
DH° = " 2H°f (NO) - 2H°f (NOCl)
22) Enounce the principle of the Chatelier or the mobile equilibrium:
When an alteration of the variable ones is provoked from the outside (P, T, concentration) of a system in equilibrium, the equilibrium is moved in a sense that is opposed to the brought variation, stretching to diminish the effects.
23) Thing happens if it is attempted to increase the concentration of an element in the reaction:
The equilibrium constant must remain constant and therefore if it increases the concentration of a substance, the other substances of the reaction will change their concentration so as to to make that Kc remains constant, that wants to say that the system reacts so as to to consume the compound excess.
24) Which effect has a variation of the external pressure on a system in equilibrium:
It depends on the type of reaction, as an example in the reaction N2 3H2 " 2NH3 if the reaction proceeds towards dx, the n° of wharves diminishes, therefore if as an example it comes increased the external pressure on this system in equilibrium, it will try to answer so as to to cancel this increase of pressure, that it is obtained moving the equilibrium towards the n° smaller of wharves, that is it will remain small reagent part in how much nearly completely transforms to you in products of the reaction.
25) When a chemical transformation says homogenous and when heterogenous:
When it is reatti to you that produced they are present in an only phase, heterogenous if the substances in chemical equilibrium are present in 2 or more it is made.
26) As they come influenced the gaseous dissosciations from pressure and temperature:
They must be expressed in terms of the degree of dissosciation to, which it will have to change so as to to balance those that are the changes of pressure or temperature, enough of the rest to observe that the dissosciation is a reaction that happens with increase of the n° of wharves and therefore it is favorite from one diminuizione of the external pressure.
It is observed moreover that being the dissosciation characterized from breach of ties, it cannot that to be endothermic, that is with purchase of heat from the atmosphere and therefore is favorite from a temperature increase.
27) Enounce in terms of to the wharves for the reaction: 2 NOCl " 2 NOT Cl2 :
2 NOCl " 2 NO Cl2
n0(1 - to) (n0to ) (n0to)/2
eventual other wharves that were present for the reaction products, go added to the already present terms. If other reagents were present, the reaction could not be written in terms of to in how much would ignore the contribution on the products. The dissosciation only has therefore sense for these reactions where a compound scinde in 2 or more members.
28) Enounce in the reaction dissociated terms of wharves: 2 NOCl " 2 NOT Cl2 :
2 NOCl " 2 NO Cl2
n0(NOCl) - 2x n0(NOT) 2x n0(Cl2) x
29) Like estimating if one given reaction is in equilibrium and, if it it is not, in which sense passes spontaneously:
The D G =D G° RT ln Q is estimated. The following cases can be introduced:
DG = 0 in such case the reaction is in equilibrium.
DG < 0 the reaction passes spontaneously from sx towards dx.
DG > 0 the reaction passes spontaneously from dx towards sx.
In this last case an eventual calculation of the wharves previews that the wharves that they diminish regarding the situation begin them are those of the members who find themselves to dx in the reaction, while the members to sx increase the n° of wharves.
30) In equilibriums in which gases appear solid and, which it is the importance of the wharves of the solid one:
The wharves of the solid one that are consumed are in exact stoichiometric relationship with the values of the wharves that are created which must but be obtained through the equilibrium constants. As an example in the calculation of the wharves totals for the Kc, they go only considered the wharves of gases.
31) As they come influenced the equilibriums of the solid systems and liquids from modifications of the pressure or the temperature:
The pressure has effects minimums in how much in these reactions is insufficient the volume variations.
32) As one can be velocizzare reaction:
If it is reversible must itself be removed the reaction products as forms itself, in such a way the process happens in way almost completes.
33) As they come it influences the heterogenous equilibriums to you from temperature or pressure variations:
There are reactions for which being present liquids, solid and gas, it is immediate to understand in which sense the reaction is carried out with volume increase, the reaction will turn out stimulated in such sense if the external pressure diminishes.
It is intuitivo as an example to think that if the pressure is increased, the arrange-reaction will try to diminish its pressure and therefore the equilibrium will be moved so as to sfavorire the gas creation regarding solid liquids or.
34) That particularitity has equilibrio the NH4HS(s) " NH3 (S) H2S(G)
The reagent is found to the solid state therefore does not appear any in KC that in KP neither in some eventual calculation of the wharves through PV = n RT, that it only has sense for the members to the gaseous state.
35) For the equilibrium SnO2 2 H2(G) " Sn(S) 2 2HOr(g) as 5 wharves of Sn can be obtained:
Famous 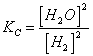 the must be made yes that [ H2 ] is such that the wharves of
H2 are 10 that is the double
quantity of the wharves of pond.
the must be made yes that [ H2 ] is such that the wharves of
H2 are 10 that is the double
quantity of the wharves of pond.
36) For the equilibrium C(s) HOr2(g) " CO(G) H2 (g) how much water is necessary to me for gasare 1.1 wharves of C(s) :
1.1 wharves of CO and 1.1 wharves of Or 2 must be created , so that that happens dve to desumere the wharves to the equilibrium from the KP and to these to add 1,1.
37) Why if lasci opened the mineral water this sgasa:
If the bottle is sluice there is of CO2 between water and stopper that has one determined pressure, if we open the bottle, this CO2 exits and the pressure to which she is subordinate the solution is the much lowest one, therefore CO 2under shape of bollicine who go up in surface, in the vain attempt from part of the solution will be produced to answer to this variation of the system therefore ends that nel.giro.di 10 minuteren the water sgasa completely.
