Solutions
1) What is one solution:
It is a formed homogenous system from 2 or more members which find themselves dispersed in the system under Ionian molecule shape or. Fundamental E' that the particles have molecular dimensions, otherwise is spoken about colloidal state.
2) You they can be chemical ties between the present substances in the solution:
Not, otherwise it would be be a matter of a new one composed. It must therefore be possible to riottenere from the solution the component substances by means of physical procedures of separation.
3) Thing characterizes and what ¨ the concentration of one solution:
It characterizes the composition and it is defined like the amount of every present member in one given to mass or volume of solution.
4) That measure unit has the concentration:
Reported to the weight:
for hundreds in peso g of solute in 100g of solution
molalità m n° wharves of solute in 1000g of dissolvent
Fraction molare x n° wharves of the member/n° wharves totals of the solution
Reported to the volume:
For hundreds in volume g of solute in 100 mililiter of solution
Molarità M n° of wharves of solute in 1000ml of solution
Normalità N n° of equivalents of solute in 1000ml of solution
![]()
For hundreds in volume mililiter of solute in 100ml of solution. the volume total of the solution is not equal to the sum of the volumes of the 2 members but inferior.
5) That relationship is between Normalità and Molarità:
![]() where to it is an entire one that it is worth 1 for
monoprotici acids, 2 for diprotici acids... and cos¬ via.
where to it is an entire one that it is worth 1 for
monoprotici acids, 2 for diprotici acids... and cos¬ via.
It is the same coefficient that alloy PM and Peq.
6) When a solution is it saturates:
When in it the solute one indisciolto is in equilibrium with the solute one disciolto.
7) When a solution is soprasatura, and its stability:
A solution is soprasatura when for it a advanced solute concentration of to the concentration correspondent to the solution is caught up saturates. He is not stable, always stretches to catch up the concentration of the solution saturates.
8) What is the solubility:
For one determined substance in a dissolvent data to one given to temperature the solubility it corresponds to the concentration of the solution saturates to that temperature, that is to how much solute one it is found disciolto in the dissolvent for that particular solution in which the dissolvent cannot disciogliersi ulteriorly.
9) What is the solubility product:
It is simply the law of action of mass reported to the reaction of ionization of the solute KS = [ To ][B-]/[ AB ] and siccome would not have to be spoken about concentrations but of activity, it achieves some that [ AB ] must be unitary and therefore
KS = [ To ][B-] = constant.
10) How much is worth the solubility of knows them Pb(IO3)2 melted in water:
Pb(IO3)2 ionizza in havingPb 2 concentration s and 32IO- having concentration 2s.
Ks = [ Pb2 ][IO3-]2 = s * (2 s)2 = 4 s3
11) Which it is the condition affinchè forms of the hasty one:
In a data moment the product [ To ][B-] it must be > of the Ks, which having to be constant it imposes that the reaction is moved towards the solute one to the solid state.
12) Describe the effect of the ione common:
The solubility of a solute one in pure water more is elevated regarding the case in which same the solute one its is disciolto in one watery solution containing ione. In order to calculate it it is necessary to consider in the solubility product that the concentration of the ione in issue more is elevated while Ks must remain constant therefore in the complex will create little quntità of the ione not common that it is equivalent to say that is melted less solute.
13) If I have a solute one on the bottom of a solution, which it is the condition affinchè is melted all:
It must be the equal solubility to the relationship between n° of wharves and the volume of the solution, in fact the solubility is the concentration of the solution in which the solute one is melted.
14) Like calculating the concentration of the Ionian ones in the solution when one of the 2 is in strong excess regarding the other:
It must be considered that the ione present with smaller concentration it falls all if [ To ][B-] > Ks, the concentration of the ione in excess obtains embezzling the amount of the other ione that it falls while the amount of the other ione that it remains in solution gains from the Ks. In practical the effect of the ione is necessary to keep in mind common.
15) Like calculating the concentration of the Ionian ones in solution when they are in the exact stoichiometric relationship:
The solution can be considered neglecting the effect of the ione common and considering therefore as it knows them disciolto in pure water.
16) What is the meaning for aliquot precipitation:
The situation agrees for which a same one ione can determine the precipitation of 2 various solute ones, in such case, the 1° to fall it is that one for which smaller concentration of this ione common is demanded one.
17) What is the division coefficient:
When a solute one is placed to contact with 2 not miscible
dissolvents, ripartisce between of they forming 2 distinguished
solutions. The division coefficient expresses exactly that the
relationship between the concentration of the solute one in the
dissolvent To and the concentration of the solute one in the B
dissolvent is constant. 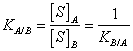
![]()
18) How many grams of dissolvent remain in solution to continuation of the extraction with V liters of dissolvent:
![]()
19) As one can be extracted from the dissolvent solute amount of greater, using little liters of according to dissolvent that not in the case of the previous question:
The extraction can be carried out consecutively more times with equal amounts of the 2° dissolvent, in such case
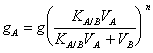
20) Enounce the law of Henry on the solubility of gases in the liquids:
It asserts that the solubility of a gas in a liquid is proporziona them to the partial pressure of the gas in equilibrium with the solution, on condition that the gas does not react with it.
21) What is the solubility coefficient:
It expresses how much gas it is melted in a liquid volume
and is independent from the pressure ![]() .
.
22) What is the absorption coefficient:
It expresses how much gas is melted in the unitary volume
to the temperature t when the pressure of the gas in equilibrium with
the solution is equal to 1 atm ![]() where VG0 is the volume
brought back to the conditions of temperature of 1 Atm and 0 K
through
where VG0 is the volume
brought back to the conditions of temperature of 1 Atm and 0 K
through ![]() .
.
23) That relation is between the coefficient of absorption to and the solubility coefficient b :
![]() where T0 = 273K
where T0 = 273K
24) In that way the nature of the dissolvent and the solute one acts on the solubility:
The process from a thermodynamic point of view in 3 must be analyzed is made:
a) Comes separated molecules of soluto(endotermico).
b) Comes separated molecules of solvente(endotermico).
c) Comes forms ties to you between molecules of solute and solvente(esotermico)
Energetic budget of these 3 is made implies the different solubility, in particular a solute data is melted in the dissolvents whose molecules are estates joined from the same type of ties intermolecolari(forze of van der Waals and dipolar moments) present in solute the pure one.
25) What is the solution entalpy and to what it serves:
Inner Energy PV is the variation of entalpy (H =) associate to the process of creation of the solution. It is useful in order to determine if the solubility of that date solution increases or diminishes with the temperature, in the fattispecie, the temperature increases if the process is endothermic (H > 0), diminishes if the process is exothermic (H < 0).
26) That effect has the pressure on the solubility:
Increasing the pressure the volume diminishes and therefore it consequently increases to the concentration and the solubility.
27) To what it serves and that characteristic ideal solution has one:
Servants to study the numerous phenomena that interest the solution process. Its characteristics are:
to) the volume of the solution he is equal to the sum of the volumes of the members.
b) the formation of the solution to leave from the members is not accompanied from development or absorption of heat.
28) What is and which are the colligative property of the ideal solutions:
They are property that only depend on the particle number of solute contained in the unit of solution volume and therefore completely independent from the property chemistries of the members. They are:
a) When in the liquid comes disciolto a solute one, the vapor tension comes modified.
b) crioscopico Lowering
c) ebulloscopico Elevation.
d) osmotica Pressure.
29) What is the vapor tension and like it comes modified to continuation of the introduction of the solute one in the dissolvent:
The vapor tension is the pressure for which the liquid is in equilibrium with its vapor to one determined temperature, it that is indicates just the pressure that competes to the vapor, remembering that a liquid coexists with its vapor in the course of a state passage, transformation for which the pressure remains constant for one given temperature.
If I insert a not soluble dissolvent, it distacca therefore to one given temperature does not have the equilibrium for one pressure and therefore lower vapor tension.
30) Enounce the law of Raoult:
The relative lowering of the vapor tension of the dissolvent is equal to the fraction molar of the solute one
![]() Valid only if the solute one is one substance
not volatile.
the same concept in other words:
Valid only if the solute one is one substance
not volatile.
the same concept in other words:
The vapor tension of a given ideal solution from the members To and B is equal to the sum of the products of the vapor tensions of the single members to the pure state for the respective fraction molar in the solution.
P = P0To * XTo P0B * XB
If the vapor tension of a solute one, as an example B, is negligible to the considered temperature then
P = P0To * XTo
31) In the calculation of the lowering of the vapor tension as account of the dissosciation degree is kept:
![]()
32) as the composition of the phase is estimated vapor in fractions molars:
For the single member it is the relationship between its vapor pressure in the mixture and the pressure of vapor total of the same mixture.
33) If I have a liquid mixture in equilibrium with its vapor, which relations tie the 2 are made:
to) the vapor pressure of the single member in the vapor he is equal to the vapor tension of the member to the pure state for its fraction molar.
b) the n° of wharves of the single member in the vapor it can be gained from the PV = nRT considering the pressure that competes to the single element and that it is obtained in the point to).
34) What is the fusion or ices point:
It is the temperature to which the vapor tensions of the liquid and the solid one have the same value.
We have inasmuch as the vapor tension of the solution is inferior to the vapor tension of the dissolvent to the pure state and therefore if this vapor tension decreases, while the vapor tension of solid then the equilibrium between the two vapor tensions remains constant must necessarily happen for one lower temperature.
35) Describe the phenomenon of the crioscopico lowering:
The ices point of the solution regarding the ices point of the pure dissolvent is lowered. Dcr = Kcr * molalità
36) Explain why it is thrown knows them on the mountain roads in the winter periods:
It knows them are characterizes you from the fact that is completely dissociates to you, therefore in the case minimal (as an example NaCl) the factor of Van' t Hoff is equal to 2. that means that DCR = KCR * molalità * 2, therefore does not make other that to lower the threshold to which the water it freezes, avoiding in such a way the ice formation.
37) What is the point of boiling of a liquid:
It is the temperature to which the vapor tension of the liquid equals the external pressure.
We have seen that the vapor tension della solution is inferior alla dissolvent vapor tension del allo be pure and therefore if this vapor tension decreases it is necessary one higher temperature in order to equal the external pressure.
38) Describe the phenomenon of the ebulloscopico elevation:
The point of boiling of the solution regarding the ices point of the pure dissolvent is raised. Deb = Keb * molalità
39) Explain why, when it is thrown of knows them in the water that bubbles, this seems to calm itself:
It knows them are characterizes you from the fact that is completely dissociates to you, therefore in the case minimal (as an example NaCl) the factor of Van' t Hoff is equal to 2. that means that Deb = Keb * molalità * 2, therefore does not make other that to raise TEBOLLIZIONE .
40) What is one semipermeabile membrane:
It is a wall that leaves to pass to the dissolvent molecules but those of solute not concurring to measure of the osmotica pressure from this exercising.
41) That what ¨ the osmotica pressure:
It is the tendency of molecules of solute expanding itself in all the volume of dissolvent to disposition.
Its expression is similar to that one of the ideal gases p V = n R T where n it is the n° of wharves of solute while p, V and T varying of this formula that is osmotica P p = c R T refers to the soluzione. Is possible also where c = n/V.
42) To what the fact has had that the osmotica pressure is expressed with a law similar to that one of ideal gases:
It measure in way of the all analogous tendency of dissolvent molecules to expand in all the volume of solution to they disposition.
43) That relationship is between the osmotica pressure p and the unevenness in a branch of a tube to U:
p = h (cm) * density (g/cm3) * 981 (dine) = ........ dine/cm2 = [ (.......... dine/cm2 )/1,013*106 ] atmospheres
44) For a solution of HNO3 in water, it is necessary to consider the binomial of Van' t Hoff:
It is necessary to consider it in the case are given us property relati you to whichever colligativa, in how much HNO3 it is a strong acid and therefore completely dissociated, therefore the factor for which they go multiplied all the colligative property is not worth 1 but 2 and cannot therefore be omitted.
45) How much is worth the osmotica pressure for one solution of 2BaCl and KCl:
p = CTOT * R * T
where CTOT = C(BaCl2) * i (BaCl2) C(KCl) * i (KCl) = C(BaCl2) * 3 C(KCl) * 2
46) When 2 solutions are isotoniche:
When they have the same osmotica pressure.
