Batteries
1) In how many ways the passage of electrons from the shape reduced of one is possible conjugated brace to the shape oxidized of the other:
to) Directly, stirring the containing solutions the 2 braces conjugated of ossidoriduzione, in this case the elettroni pass from a molecule to the other quickly, it is not possible to gain of some job electrical worker and lâequilibrio comes moreover caught up quickly.
b) Indirectly in an electrochemical device which the battery that concurs to take advantage of the job electrical worker generated from the electron flow that comes convogliato in a small municipal and therefore the reaction can last to along.
2) Write the reaction of reduction of copper:
Cu2 2e ® Cu(s)
3) Write the reaction of oxidation of copper:
Cu(s) ® Cu2 2e
4) What is one battery:
A battery is an electrochemical device that concurs to use the job electrical worker developed in the course of the reactions of ossidoriduzione by means of separation of the solution that is reduced from that it is oxidized and they connection through electrodes only connects you to the utilizzatore circuit. The circuit comes closed through a salt bridge that concurs the transfer of the Ionian ones but the physical union of the 2 solutions does not concur.
5) In that way comes described the generic semielement:
Elettrodo | forms oxidized: reduced shape |
where the oxidized shape is that one that it more has n° of elevated oxidation.
6) That electrode is used if it is the oxidized shape that the reduced shape is to the state of solution:
An electrode of an inert metal is used which the Pt platinum. In this case it comes said electrode to ossidoriduzione.
7) That electrode is used when the reduced shape is a metal:
An electrode constituted from the same metal is used. It comes called electrode of 1ª species.
8) What is an electrode of 2ª species:
It is an electrode formed from a metal dipped in a solution saturates of its knows them little soluble, in the solution is disciolto moreover a containing electrolyte the anion of knows them little soluble.
As an example the electrode: Ag | AgCl(s) |
| HCl (0,1M) |
where the reactions sono Ag e " Ag(s)
e AgCl(s) e " Ag Cl- therefore the total reaction is:
AgCl(s) e " Ag(s) Cl-
9) That electrode is used if the oxidized shape or the reduced shape is to the gaseous state:
An electrode of an inert metal is used which the Pt platinum and on it is made gorgogliare the gas. It comes called electrode to gas.
10) As one is outlined battery:
Simply joining the rappresentazioni of its 2 semielements.
11) In a battery which semielement behaves itself from cathode and which from anode:
The electrode near which the oxidation happens involves from anode from the Greek word that it means towards the high, it is to upgrades them more bottom regarding the cathode, that is the other electrode near which the reduction reaction happens, cathode derives from the Greek word that it wants to say towards the bottom, is found to upgrades elevated them more so as to to attract electrons it develops to you in the oxidation of the anode.
12) What is the electromotive force of one battery:
It is the potential difference measured them to the heads of the 2 electrodes to open circuit. And = and CATHODE - andANODE , it is always positive in how much the cathode is found to upgrades elevated them for definition more.
13) What is a normal electrode:
It is an electrode in which all the activities of the substances interested to the semireaction are unitary, that wants to say that
to) the substances in solution they must have concentration 1M
b) the substances to the gaseous state must have the pressure of 1 atm.
14) What is upgrades them normal of an semielement:
It is upgrades them of a normal electrode, E° CU 2 /CUis indicated as an example it represents as all the functions upgrade them a function of state that therefore only depends on the state begins them and final and is esprimibile only in terms of a variation, therefore have been necessary to choose upgrade them of reference E°H /H2 = 0 v.
15) Describe the electrode of reference NHE:
Sheet of Pt in contact with gaseous H 2 to the pressure of 1 atm is the normal electrode to hydrogen formed from one and dipped in one solution of H3Orhaving unitary activity.
16) What is the meaning with the acronym WAKES:
S' means Standard Electrode Calomel, or electrode standard of 2ª species to calomel.
17) For a reversible chemical process which that realizable one in a battery through the potenziometrico method, how much is worth the exchanged heat and how much it is worth the useful job:
The exchanged heat is Q = TDS while the useful job is LU = -DG
18) For an irreversible chemical process which that realizable one through miscelazione of 2 solutions of reatti to you, how much is worth the exchanged heat and how much it is worth the useful job:
The exchanged heat is Q = DH while the useful job is LU = 0..
19) As to calculate it upgrades them of a data semielement:
The formula of Nernst is used ![]() where
n it is the n° of electrons exchanges to you, to it indicates the
activity elevated to the correspondent stoichiometric coefficient.
where
n it is the n° of electrons exchanges to you, to it indicates the
activity elevated to the correspondent stoichiometric coefficient.
20) What is upgrades them of reduction, it upgrades them of oxidation and that tie is between the 2 it upgrades them:
It upgrades them of reduction is that associate to the reaction of reduction of one determined ossidoriduttiva brace while she upgrades them of connected oxidation is that one to the reaction of oxidation of the same brace, they are equal in module but of opposite sign.
21) As currency which it is the anode and which the cathode for one battery:
The reactions of reduction for both are written the electrodes and if it calculates some upgrades them of reduction, that most positive one is associated to the cathode where the reduction reaction will have to happen and therefore the reaction is spontaneous in the sense in which it is written, the other electrode is the anode, on it the oxidation will happen and therefore the reaction is spontaneous in the opposite back to that one in which currently it is written, after all the reaction of spontaneous ossidoriduzione is the sum of the reaction del 1° electrode for the inverse reaction of 2° the electrode.
22) What is one battery to concentration:
It is a battery in which the 2 semielements are identical while they change the activities is worth to say can be introduced:
to) various Concentrations; ![]()
b) Pressures diverse ; 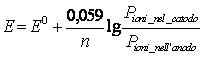
23) How much is worth the variation of free energy standard for one whichever reaction of ossidoriduzione:
DG° = - n F DE°
con DE° = E°CATODO - E°ANODO where who is the anode and who is the cathode gains from the observation which species is reduced in the back in which the reaction is written, such species will figure to the cathode.
With constant F of equal Faraday to 96500 Coulomb obtains a DG° expressed in Joule/Size, in order to pass it to Kcal/Size is necessary to divide for 4184.
24) Like estimating the spontaneità of one ossidoriduttiva reaction:
It is necessary to calculate 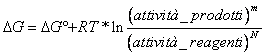 where
to the usual the activity corresponds to the pressure for gases and
the concentration for the solutions while it is unitary for the
members to the state of solid liquid or.
where
to the usual the activity corresponds to the pressure for gases and
the concentration for the solutions while it is unitary for the
members to the state of solid liquid or.
DG° is calculable dalla DG° = - n F DE° dove DE° = E°CATHODE - E°ANODO .
25) How much is worth the constant of equilibrium for one any reaction of ossidoriduzione:
![]() that to 25° expressed in terms of logarithms decimates
becomes them
that to 25° expressed in terms of logarithms decimates
becomes them ![]()
26) Which concentrations it is necessary to consider in the calculation of the Kc of one battery:
The Kc is reported to the relative concentrations to the equilibrium therefore is necessary to consider the battery unloads that is having the two electrodes to the same one upgrades them.
27) How much is worth R in the DG° = - RT ln K:
Joule Is worth 8.31/Size.
28) Thing happens to a battery that it distributes 1 Ampere for 4 hours:
1 Ampere for 4 hours means to distribute 1 Ampere * 4 * 3600sec = 14400 Coulomb, data that the distribution of 96500 Coulomb previews the passing of a size of substances acide, achieves some that scomparranno 14400/96500 wharves of substances acide because of the process of reduction and as many instead if they will form some of the oxidized shape.
29) the substance wharves that are reduced to the cathode are equal to the wharves that are oxidized to the anode:
Not, it must set up the proportion individually why as an example to every size of Ca2 2 electron wharves correspond while " size of Ag corresponds one electron size.
30) As the spontaneous ossidoridutivo process is deduced:
The electrodes are written for both the reduction reactions, are estimated upgrade associated them to the single reactions, the electrode to upgrades elevated them more is the cathode and in it a reduction therefore as already it is written, while in the anode happens spontaneously happens the oxidation and is necessary therefore to write the inverse reduction. The reaction of spontaneous ossidoriduzione is the sum of the 2 written semireactions therefore.
31) Thing happens to a cathode, in presence more ossidoriduttive braces:
The ossidoriduttiva brace is reduced with upgrades them more normal elevated.
32) Which concept alloy the batteries to the solubility product:
Through the solubility product it is succeeded to calculate the concentration of a determined one ione that sometimes it is much profit for the determination of upgrades them of the electrode through the equation of Nernst.
33) What is a storage cell to the lead:
It is the battery of the machine, a battery characterized from the fact that the products of the reactions that happen to the electrodes remain attack you to the same electrodes therefore if one equips continuum flow to the battery through the phenomenon of electrolysis pu² to bring back the battery to the conditions begins them.
